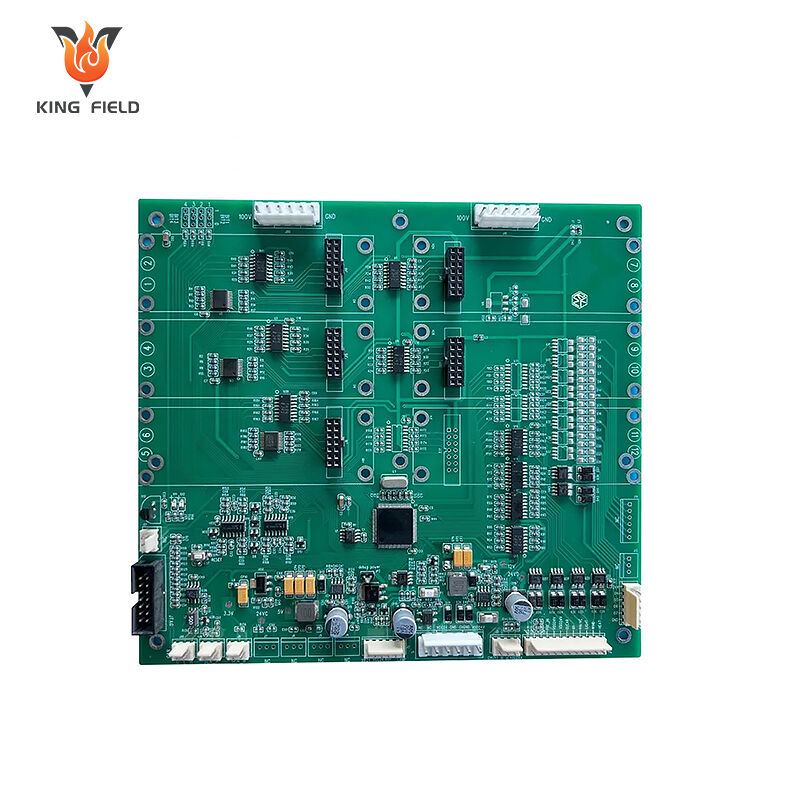
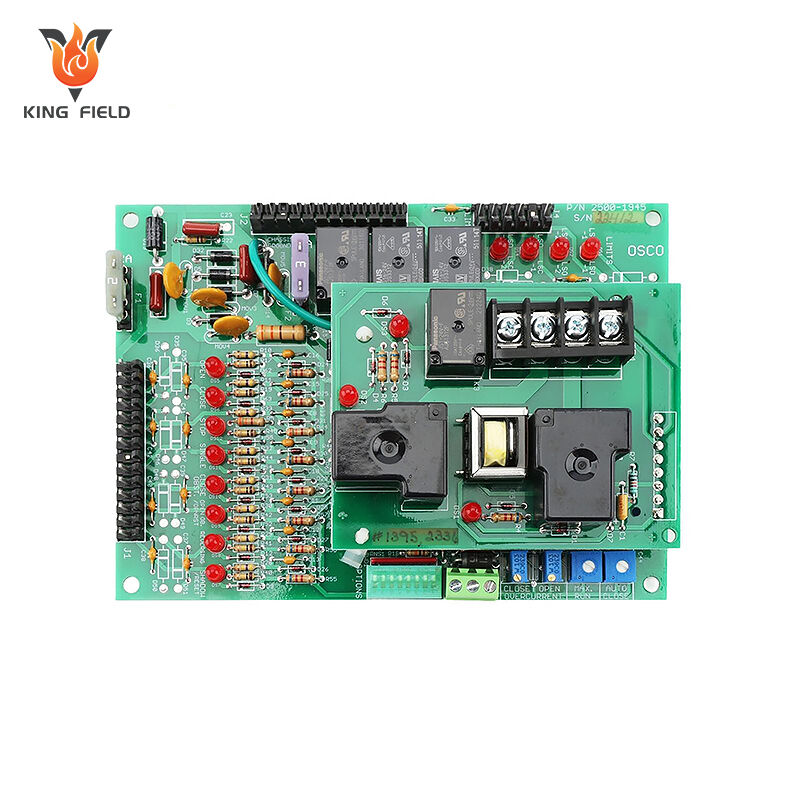
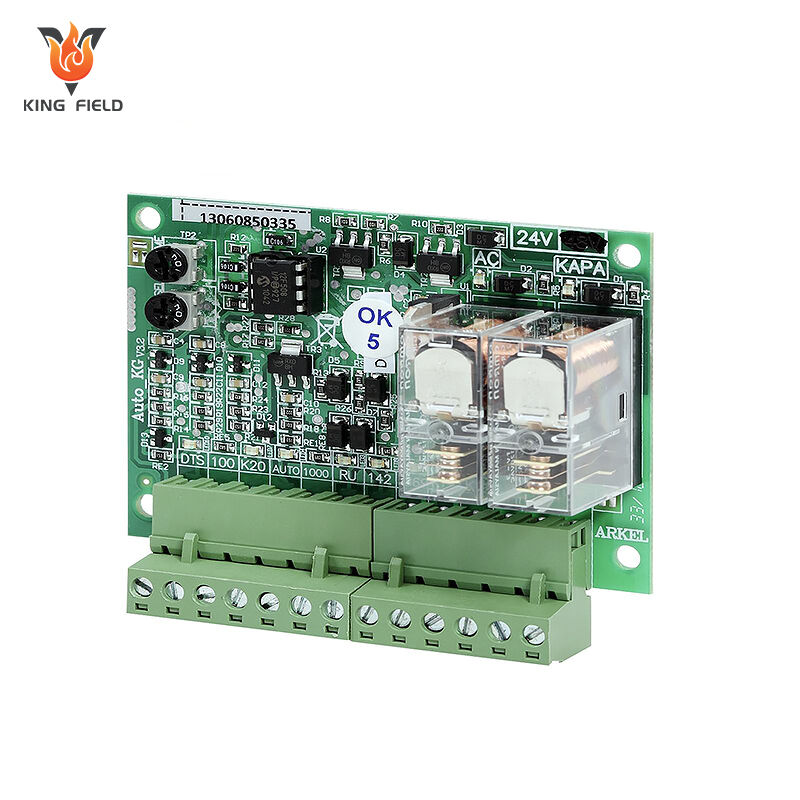
Medical PCBA
High-reliability Medical PCBA solutions engineered for compliance and critical healthcare applications. Precision assembly, biocompatible materials, strict sterilization compatibility, and multi-stage testing ensure safety and performance. From diagnostic devices to wearable medical tech—24h prototyping, fast delivery, and DFM optimization tailored to medical industry demands.
✅ ISO 13485/FDA compliant
✅ Sterilization-compatible designs
✅ Critical application reliability
✅ 24h prototyping + quality-focused assembly
Description
Overview of Medical PCBA

Medical PCBA is a core electronic component designed and manufactured specifically for medical devices. It is the "brain" of medical electronic devices, undertaking key functions such as signal processing, data transmission, and control execution. It is widely used in various medical scenarios such as diagnosis, treatment, monitoring, and rehabilitation.
Core features
• High Reliability: The operation of medical equipment is directly related to patient safety and must operate stably under long-term, high-load conditions.
• Strict Compliance: It must comply with international/domestic medical electronics standards, with full traceability from design to production.
• Low-Risk Control: It must meet requirements for leakage prevention, electromagnetic interference prevention, and biocompatibility to avoid secondary harm to the human body or the equipment.
• Precise Performance: Optimized for medical scenarios, the error rate must be controlled within an extremely low range.
Typical application scenarios
• Diagnostic equipment: Control boards and signal processing boards for ultrasound machines, CT scanners, MRI machines, blood analyzers, and nucleic acid testing instruments;
• Monitoring equipment: Core control and data transmission boards for electrocardiogram monitors, blood pressure monitors, pulse oximeters, and blood glucose meters;
• Treatment equipment: Driver boards and power control boards for ventilators, defibrillators, infusion pumps, and laser therapy instruments;
• Implantable devices: Miniature, high-reliability PCBAs for pacemakers and cochlear implants;
• Rehabilitation equipment: Motion control boards and sensor signal processing boards for rehabilitation robots and physiotherapy instruments.
Key manufacturing requirements
• Material Selection: Prioritize the use of medical-grade environmentally friendly materials; implantable products must meet biocompatibility standards.
• Process Control: Employ high-precision SMT surface mount technology and lead-free soldering processes, strictly controlling solder joint quality and product cleanliness.
• Testing and Verification: Conduct high and low temperature tests, vibration tests, EMC tests, and lifespan tests to ensure product stability and safety in complex medical environments.
• Traceability System: Establish a full-process traceability mechanism to meet the compliance review requirements of the medical industry.

Unique Challenges of Medical PCB Assembly
The core challenges of medical PCB assembly revolve around three main areas: compliance, reliability, and security, compounded by the specific requirements of the medical scenario, as follows:
1. Compliance and traceability pressures
Multiple international and domestic standards must be met, and the entire process from design to delivery must comply with the requirements of a medical electronics quality management system.
Strict traceability requirements are in place: raw material batches, production process parameters, testing data, and operator information must be recorded throughout the entire process, supporting full lifecycle traceability and meeting compliance review and recall requirements.
Material compliance restrictions are in place: lead-free, environmentally friendly, and medical-grade materials must be used. Implantable products require additional biocompatibility certification, and any potentially harmful substances are prohibited.
Strict regulatory and certification requirements necessitate that medical PCB assembly comply with a series of complex regulations and standards, including:
• FDA regulations (21 CFR Part 820, Quality System Regulations),
• ISO 13485 Medical Device Quality Management System,
• IPC standards in the electronics manufacturing field, and regional certifications.
2. High reliability requirements under extreme environments
• Environmental adaptability challenges: Medical devices need to cope with diverse operating conditions, and PCBs must withstand extreme conditions such as a wide temperature range of -40℃ to 85℃, long-term vibration, and high humidity;
• Long lifespan and zero failure requirements: Monitors, ventilators, and other equipment need to operate 24 hours a day without interruption, and implantable devices need to have a lifespan of 5-10 years without any risk of failure;
• Miniaturization and high-density assembly pressure: Portable medical devices and implantable devices have stringent requirements for PCB size, requiring ultra-fine pitch and micro-pad assembly, which can easily lead to problems such as bridging, cold solder joints, and poor heat dissipation.
3. Electrical safety and electromagnetic compatibility challenges
• Electrical safety protection: Medical electrical equipment must meet the requirements for protection against electric shock and leakage. PCB layout must strictly distinguish between high-voltage and low-voltage areas to avoid the risk of insulation breakdown.
• High difficulty in meeting EMC performance standards: Medical equipment itself generates strong electromagnetic interference and must also resist external environmental interference. PCBs must pass EMC testing, and grounding design, shielding structure, and filtering circuits must be optimized to avoid interference causing data distortion or equipment malfunction.
• Signal integrity requirements: Diagnostic equipment needs to transmit high-precision analog signals. PCB assembly must control impedance matching, reduce signal attenuation and crosstalk, and ensure the accuracy of data acquisition and transmission.
4. Challenges in Cleanliness and Process Control
• High cleanliness requirements: Medical devices have extremely high requirements for PCB cleanliness. Contaminants such as solder slag, flux residue, and dust may cause short circuits or trigger reactions in human tissues. Production must be carried out in a Class 1000 cleanroom, and residues must be removed after assembly through processes such as ultrasonic cleaning and ion cleaning.
• Difficulty in precision process control: The use of micro-assembly technology and lead-free soldering requires strict control of reflow soldering temperature profiles and mounting pressure to avoid defects such as tombstoning, cold solder joints, and voids in solder joints.
• Pressure of small-batch, multi-variety production: Medical devices are mostly customized, with small-batch orders. It is necessary to quickly switch product models while ensuring consistency between different batches of products, which places extremely high demands on the flexibility of the production line and the stability of the process.
5. Stringent testing and verification requirements
• Full inspection, not random sampling: No defective medical PCBs are allowed to enter the market. Every PCB must be 100% inspected, with 100% inspection coverage.
• Long reliability verification cycle and high cost: PCBs must pass high and low temperature cycling tests, damp heat aging tests, vibration tests, and lifespan tests, with verification cycles lasting several months.
• Special scenario verification: Implantable PCBs require additional biocompatibility and body fluid corrosion tests, while diagnostic PCBs require signal accuracy calibration tests to ensure compliance with clinical use requirements of medical devices.
Manufacturing capacity
| Equipment manufacturing process capability | |||||
| SMT Capacity | 60,000,000 chips/day | ||||
| THT Capacity | 1,500,000 chips/day | ||||
| Delivery time | Expedited 24 hours | ||||
| Types of PCBs Available for Assembly | Rigid boards, flexible boards, rigid-flex boards, aluminum boards | ||||
| PCB Specifications for Assembly | Maximum size: 480x510 mm; Minimum size: 50x100 mm | ||||
| Minimum Assembly Component | 01005 | ||||
| Minimum BGA | Rigid boards 0.3 mm; Flexible boards 0.4 mm | ||||
| Minimum Fine-Pitch Component | 0.2 mm | ||||
| Component Placement Accuracy | ±0.015 mm | ||||
| Maximum Component Height | 25 mm | ||||
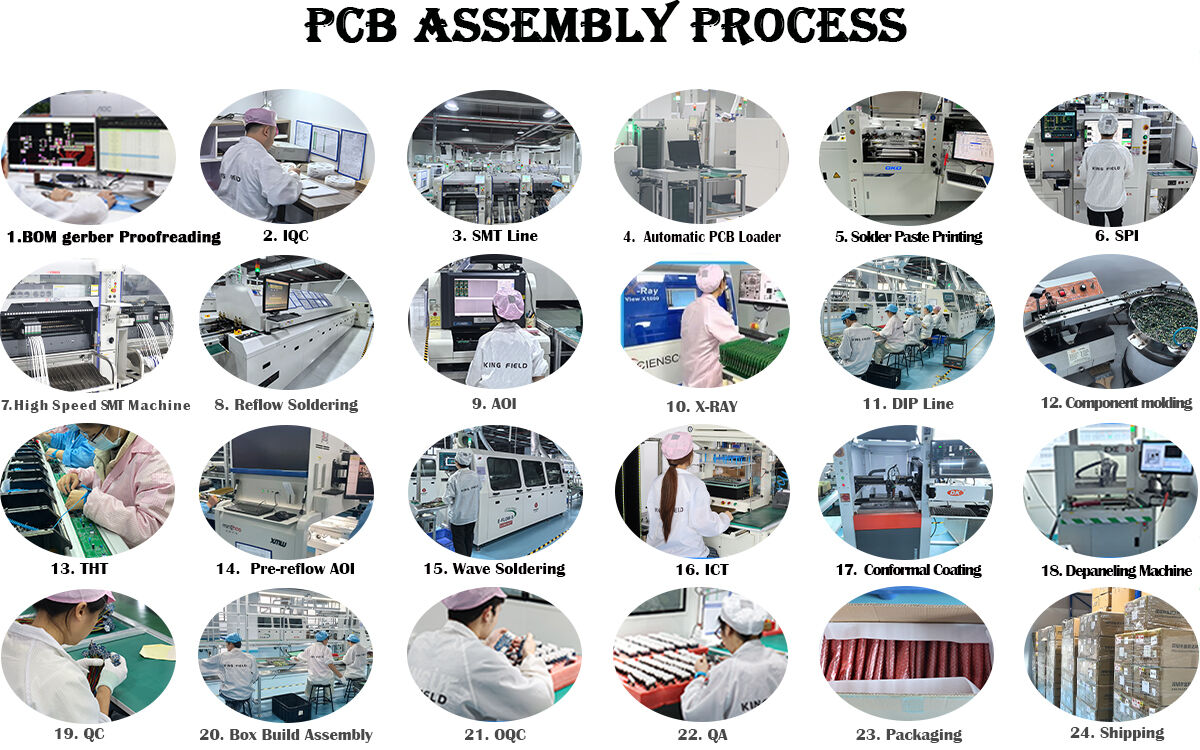
Core Advantages
Kingfield Medical PCBA Solutions – Empowering Innovation in High-Reliability Medical ElectronicsCore positioning
We provide compliant, highly reliable, and safe PCB assembly services specifically for diagnostic, therapeutic, monitoring, and implantable medical devices. With the ISO 13485 full-process quality control system at our core, we create core medical electronic components that meet international standards such as FDA and IEC 60601, helping medical device companies overcome technological barriers and ensuring safe clinical use.
Comprehensive compliance assurance, meeting the stringent standards of the medical industry
• System Certification: Certified by ISO 13485 Medical Quality Management System and RoHS/REACH Environmental Protection Certification; products comply with IEC 60601 Medical Electrical Safety Standard and ISO 10993 Biocompatibility Requirements;
• Full-Process Traceability: Raw material batches, production parameters, testing data, and operator records are kept throughout the entire process, supporting full lifecycle traceability of products and meeting FDA QSR compliance review and recall requirements;
• Material Compliance: Strictly selects medical-grade lead-free environmentally friendly materials; implantable products use biocompatible substrates and solders, eliminating the risk of hazardous substances.
Adaptable to extreme environments, highly reliable and long-life design
• Environmental tolerance: Supports wide temperature range of -40℃ to 85℃, withstands high humidity of 95% RH and vibration shock of 10-2000Hz, and is suitable for complex scenarios such as operating rooms, ambulances, and human implantation;
• Long lifespan guarantee: MTBF ≥ 100,000 hours, implantable products have a lifespan of 5-10 years, and the zero-failure design meets the 24-hour uninterrupted operation requirements of medical devices;
• High-density assembly: Proficient in 01005 packaging and BGA ultra-fine pitch assembly below 0.4mm, supporting the precision manufacturing of miniaturized and portable medical devices.
Both safety and performance standards are met, eliminating clinical risks
• Electrical Safety: Class I/II insulation design, leakage current <100μA, strong current/weak current zoned layout to avoid electric shock risk;
• EMC Optimization: Passed IEC 61000 electromagnetic compatibility test, optimized grounding, shielding and filtering design to eliminate interference between devices and ensure accurate diagnostic data;
• Signal Integrity: Strictly controlled impedance matching (±10%) to reduce crosstalk and signal attenuation, adapting to the signal transmission requirements of high-precision equipment such as CT scanners and nucleic acid testing instruments.
Refined process control ensures product consistency
• Clean Production: Class 1000 cleanroom with ultrasonic cleaning and ion cleaning processes, resulting in near-zero solder slag and flux residue;
• Precision Manufacturing: SMT placement accuracy ±0.03mm, reflow soldering temperature profile difference ±2℃, solder joint void rate <5%, supporting flexible production of small batches and multiple varieties;
• Full Inspection Guarantee: 100% AOI visual inspection, X-ray solder joint inspection, ICT circuit testing, FCT functional testing, with defect rate controlled at the PPM level.